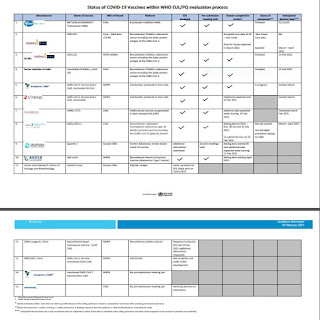
WHO အသိအမှတ်ပြု ကိုဗစ်ကာကွယ်ဆေးများစာရင်း
• The SII/Covishield and AstraZeneca/AZD1222 vaccines (developed by AstraZeneca/Oxford and manufactured by the State Institute of India and SK Bio respectively) were given EUL on 16 February.
• The Janssen/Ad26.COV 2.S developed by Johnson & Johnson, was listed for EUL on 12 March 2021.
• The Moderna COVID-19 vaccine (mRNA 1273) was listed for EUL on 30 April 2021 and the Sinopharm COVID-19 vaccine was listed for EUL on 7 May 2021.
• The Sinopharm vaccine is produced by Beijing Bio-Institute of Biological Products Co Ltd, subsidiary of China National Biotec Group (CNBG).
• The Sinovac-CoronaVac was listed for EUL on 1 June 2021.
1. Pfizer BioNtech
2. AstraZeneca
3. SK BIO AstraZeneca
4. Serum Institute of India
5. Sinopharm / BIBP
6. Sinovac
7. Moderna
8. Janssen
9. The Gamaleya
10. CanSinoBIO
11. Vector State Research Centre of Virology and Biotechnology
12. Zhifei Longcom, China
13. IMBCAMA, China
14. Sinopharm / WIBP
15. NOVAX
ဒေါက်တာတင့်ဆွေ



Comments
Post a Comment